|
Introduction
Neurodevelopment and Adaptation to a Violent World
Cortical Modulation and the Use-dependent Development of the Brain
The Child s Response to Threat
The Hyperarousal Continuum: Fight or Flight Responses
Neural Systems Regulating the Neurobiological Response to Threat
Reticular Activating System (RAS)
Locus Coeruleus
Hippocampus
Amygdala and Emotional Memory
Hypothalamic-Pituitary-Adrenal Axis (HPA)
The Dissociative Continuum
Neurobiology of dissociation
States become Traits: The Clinical Presentation of Children Exposed to Violence
Altered Neurobiology in Children Exposed to Violence
Clinical Implications
A. Use-dependent Learning: State Dependent Storage and Recall
The Future: Impediments to Problem-Solving and Prevention
References
Tables and Figures
Introduction
We humans are the most complex and puzzling of living creatures. We can create, nurture, protect, educate and enrich. Yet we also degrade, humiliate, enslave, hate, destroy and kill. A man can tenderly hold his newborn and moments later beat the baby s mother. Violence permeates our history. In all societies and in each culture, past and present, violence has played a role in shaping our sociocultural evolution. While no society has been able to break free from violence, there is tremendous variation in the type and degree of violence across cultures and time. In some cultures, random street violence has been suppressed with oppressive institutional violence, in others, inter-familial violence is rare but intra-familial violence violence to wives and children is rampant.
Today, in the United States, despite remarkable advances in technology, social justice, and education, violence continues to be a permeating and pervasive element of American society. We are bathed in violent images. Violence fascinates and repulses us. Whether journalist, producer, politician or scholar, we consider, comment on and analyze violence. We have academic conferences, Congressional hearings, special documentaries - we issue opinions, create task forces, start programs, blame guns, blame Hollywood, blame parents. Yet no simple solutions emerge. We continue to be shocked, enraged and confused by the horrors of violence in our homes, schools and streets.
How can we truly begin to understand the heterogeneity and complexity of the violence that surrounds us random violence and institutionalized violence, the violence in behaviors, the violence in ideas, the violence in words? Can we ever understand the detached adolescent killing his classmates in school, mothers killing their infants, husbands killing wives, children and themselves? Can we understand random bombing of civilians in the name of God? Can we understand systematic or institutionalized rape, torture, slavery, and genocide?
Violence and its associated factors are complex and multidimensional. The present chapter will consider only one of many perspectives from which to examine violence: the impact of violence and fear on the development of the child. More specifically, violence-related neurodevelopmental changes and functional consequences of these alterations in the brain will be reviewed. This view is presented with the hope that some of devastating cost of violence to the individual child, family, community and society can be illustrated from a neurodevelopmental perspective.
Violence in Childhood: Scope of the Problem
Violence in the Home
Childhood is a dangerous time. For infants and children, survival is dependent upon adults, most typically, the nuclear family. It is in the family setting that the child is fed, clothed, sheltered, nurtured and educated. Unfortunately, it is in the familial incubator that children are most frequently manipulated, coerced, degraded, inoculated with destructive beliefs and exposed to violence.
The home is the most violent place in America (Straus, 1974). In 1995, the FBI reported that 27% of all violent crime involves family on family violence, 48% involved acquaintances with the violence often occurring in the home (National Incident-Based Reporting System, Uniform Crime Reporting Program, 1999). Children are often the witnesses to, or victims of, these violent crimes.
Violent crime statistics, however, grossly underestimate the prevalence of violence in the home. It is likely that less than 5% of all domestic violence results in a criminal report. Intra-familial abuse and domestic battery account for the majority of physical and emotional violence suffered by children in this country (see Koop et al., 1992; Horowitz et al., 1995; Carnegie Council on Adolescent Development, 1995). This violence takes many forms. The child may witness the assault of her mother by father or boyfriend. The child may be the direct victim of violence - physical or emotional - from father, mother or even older siblings. Straus and Gelles (1996) have estimated that over 29 million children commit an act of violence against a sibling each year. The child may become the direct victim of the adult male if he or she tries to intervene and protect mother or sibling. While these all cause physical violence, an additional destructive element of this intra-familial toxicity is emotional violence - humiliation, coercion, degradation, and threat of abandonment or physical assault.
Media Violence
In homes where no physical or emotional violence is present, children are still bathed in violent images; the average child spends more than three hours a day watching television. Television, videogames, music and film have become increasingly violent (Donnerstein et al., 1995). Huston and colleagues have estimated that the average 18 year old will have viewed 200,000 acts of violence on television (Huston, et al., 1992). Even with solid emotional, behavioral, cognitive and social anchors provided by a healthy home and community, this pervasive media violence increases aggression and antisocial behavior (Lewis et al., 1989; Myers et al., 1995; Mones, 1991; Hickey, 1991; Loeber et al., 1993; O'Keefe, 1995), contributes to a sense that the world is more dangerous than it is (Gerbner, 1992) and desensitizes children to future violence (Comstock and Paik, 1991). In children exposed to violence in the home, these media images of power and violence are major sources of cultural values, reinforcing what they have seen modeled at home.
Community and School Violence
There has been a dramatic increase in juvenile violence over the last 10 years. From 1986 to 1996 there was a 60% increase with juveniles now accounting for 19% of all violent crime (Snyder, 1997). Much of this is youth on youth violence. The violence in communities witnessed by youth has become so pervasive in some communities that in some studies, over half of all children surveyed had witnessed some form of violence in the year prior to the survey (Taylor et al., 1992; Richters & Martinez, 1993; Horowitz et al., 1995). The most heinous violence in schools has been widely publicized with the series of school shootings from 1992 to 1999. Yet the more common forms of school violence are intimidation, threat and simple assault. For thousands of children, school is not safe. It has been estimated that more than 250,000 students are attacked in school each month (Garrity, et al., 1994). For too many, school is a place of fear, dominated by the potential for harm and a sense of pervasive threat.
Neurodevelopment and Adaptation to a Violent World
Millions of children are victims of, or witness to, violence in the home, community or school (see Perry, 1997; Chapters 21 and 22, this volume). While the majority of homes, communities and schools are safe, far too many children experience violence in one or more of these settings. For some children, a safe community and school may help buffer the impact of violence in the home. The highest-risk children, however, are safe no where; their home is chaotic and episodically abusive, their community is fragmented and plagued by gang violence and the schools are barely capable of providing structure and safety from intimidation and threat, let alone education. These children must learn and grow despite a pervasive sense of threat. These children must adapt to this atmosphere of fear. Persisting fear and the neurophysiological adaptations to this fear can alter the development of the child s brain, resulting in changes in physiological, emotional, behavioral, cognitive and social functioning. The core principles of neurodevelopment provide important clues about the mechanisms underlying the observed functional changes in children exposed to violence.
Cortical Modulation and the Use-dependent Development of the Brain
As the brain grows and organizes from the "inside-out" and the "bottom-up" the higher, more complex areas begin to control and modulate the more reactive, primitive functioning of the lower parts of the brain (see Figure 1). The person becomes less reactive, less impulsive, more thoughtful. The brain s impulse-mediating capacity is related to the ratio between the excitatory activity of the lower, more-primitive portions of the brain (brainstem and diencephalon; see Figure 1) and the modulating activity of higher, sub-cortical and cortical areas. Any factors that increase the activity or reactivity of the brainstem (e.g., chronic traumatic stress) or decrease the moderating capacity of the limbic or cortical areas (e.g., neglect, brain injury, mental retardation, Alzheimer s, alcohol intoxication) will increase an individual s aggression, impulsivity and capacity to be violent (see below).
A key neurodevelopmental factor determining this moderating capacity is the brain s amazing capacity to organize in a use-dependent fashion. Meaning that the more any neural system is activated, the more it will change. The more a child practices piano, the more she will "build in" the motor-vestibular neural systems mediating this behavior, and, of course, the better she will become at playing piano. When an infant or toddler is spoken to as an infant and toddler, the neural systems responsible for speech and language will be "activated." Frequent and repetitive talking or singing will help the child s brain develop the capacity for language; the infant or toddler living in a setting where no one speaks or sings to them will develop language slower and may even have profound communication delays. During development, repetitive and patterned sensory experiences result in corresponding neural system organization and, thereby functioning (Courchesne et al., 1994). The brain develops functions and capacities that reflect the patterned repetitive experiences of childhood. This is true for a host of functions associated with violent behaviors.
The capacity to moderate frustration, impulsivity, aggression and violent behavior is age-related. With a set of sufficient motor, sensory, emotional, cognitive and social experiences during infancy and childhood, the mature brain develops - in a use-dependent fashion --a mature, humane capacity to tolerate frustration, contain impulsivity and channel aggressive urges. A frustrated three year old (with a relatively unorganized cortex) will have a difficult time modulating the reactive, brainstem-mediated state of arousal and will scream, kick, bite, throw and hit. However, the older child when frustrated may feel like kicking, biting and spiting, but has built in the capacity to modulate and inhibit those urges. All theoretical frameworks in developmental psychology describe this sequential development of ego-functions and super-ego which are, simply, cortically-mediated, inhibitory capabilities which modulate the more primitive, less mature, reactive impulses of the human brain. Loss of cortical function through any variety of pathological process (e.g., stroke, dementia) results in regression -- simply, a loss of cortical modulation of arousal, impulsivity, motor hyperactivity, and aggressivity -- all mediated by lower portions of the central nervous system (brainstem, midbrain). Conversely, any deprivation of optimal developmental experiences which leads to underdevelopment of cortical, sub-cortical and limbic areas will necessarily result in persistence of primitive, immature behavioral reactivity and, predispose to violent behavior.
A growing body of evidence suggests that exposure to violence or trauma alters the developing brain by altering normal neurodevelopmental processes. Trauma influences the pattern, intensity and nature of sensory perceptual and affective experience of events during childhood (see Perry, 1994; Perry et al., 1995; Perry, 1997; Perry, 1999). Threat activates the brain s stress-response neurobiology. This activation, in turn, can affect the development of the brain by altering neurogenesis, migration, synaptogenesis, and neurochemical differentiation (Lauder, 1988; McAllister et al., 1999). Indeed, the developing brain is exquisitely sensitive to stress. For example, rats exposed to perinatal handling stress show major alterations in their stress response later in life (Plotsky and Meany, 1993; Vaid et al., 1997; Valee et al., 1997). These animal models suggest that early exposure to consistent, moderate stress can result in resilience, while exposure to unpredictable or chronic stress results in functional deficits and vulnerability to future stressors.
The human brain develops and, once developed, changes in a use-dependent fashion (for review see Perry et al., 1995; Perry & Pollard, 1998; Perry, 1999). Neural systems that are activated in a repetitive fashion can change in permanent ways, altering synaptic number and micro-architecture, dendritic density, and the expression of a host of important structural and functional cellular constituents such as enzymes or neurotransmitter receptors (Brown, 1994; Courchesne et al., 1994; McAllister et al., 1999). The more any neural system is activated, the more it will modify and build in the functional capacities associated with that activation. The more someone practices the piano, the more the motor-vestibular neural systems involved in that behavior become engrained. The more someone is exposed to a second language, the more the neurobiological networks allowing that language to be perceived and spoken will modify. And the more threat-related neural systems are activated during development, the more they will become built in.
In summary, then, exposure to violence activates a set of threat-responses in the child s developing brain; in turn, excess activation of the neural systems involved in the threat responses can alter the developing brain; finally, these alterations may manifest as functional changes in emotional, behavioral and cognitive functioning. The roots of violence-related problems, therefore, can be found in the adaptive responses to threat present during the violent experiences. The specific changes in neurodevelopment and function will depend upon the child s response to the threat, the specific nature of the violent experience(s) and a host of factors associated with the child, their family and community (see Perry & Azad, 1999).
The Child s Response to Threat
When the child perceives threat (e.g., anticipating an assault on self or loved one), their brain will orchestrate a total-body mobilization to adapt to the challenge. Their emotional, behavioral, cognitive, social and physiological functioning will change. These responses to threat are heterogeneous and graded. The degree and nature of a specific response will vary from individual to individual in any single event and across events for any given individual. In animals and in humans, two primary but interactive response patterns, hyperarousal and dissociative, have been described (Perry et al., 1995; Perry, 1999). Most individuals use various combinations of these two distinct response patterns during any given traumatic event. The predominant response patterns and combinations of these primary styles appear to shift from dissociative (common in babies and young children) to hyperarousal during development.
The Hyperarousal Continuum: Fight or Flight Responses
Neural Systems Regulating the Neurobiological Response to Threat
Reticular Activating System (RAS): The initial phase of the hyperarousal continuum is an alarm reaction that begins to activate the central and peripheral nervous system. A network of ascending arousal-related neural systems in the brain consisting of locus coeruleus noradrenergic neurons, dorsal raphe serotonin neurons, cholinergic neurons from the lateral dorsal tegmentum, mesolimbic and mesocortical dopaminergic neurons, among others, form the reticular activating system (RAS). Much of the original research on arousal, fear, response to stress and threat was carried out using various lesion models of the RAS (Moore & Bloom, 1979). The RAS is an important, multi-system network involved in arousal, anxiety and modulation of limbic and cortical processing (Munk, et al., 1996). These key brainstem and midbrain monoamine systems, working together, provide the flexible and diverse functions necessary to modulate the variety of functions related to anxiety regulation.
Locus Coeruleus: A key component of the RAS network is the locus coeruleus (Murberg et al., 1990; Aston-Jones et al., 1996). This bilateral nuclei of norepinephrine-containing neurons originates in the pons and sends diverse axonal projections to virtually all major brain regions, enabling its function as a general regulator of noradrenergic tone and activity (see Aston-Jones et al., 1996). The LC plays a major role in determining the valence or value of incoming sensory information, increasing in activity if the information is novel or potentially threatening (Abercrombie & Jacobs. 1987b; Abercrombie & Jacobs. 1987b). The ventral tegmental nucleus (VTN) also plays a part in regulating the sympathetic nuclei in the pons/medulla (Moore & Bloom. 1979). Acute stress results in an increase in LC and VTN activity and release of norepinephrine that influences the brain and the rest of the body. These brainstem catecholamine systems (LC and VTN) projecting to all key areas of the brain, then, play a critical role in regulating arousal, vigilance, affect, behavioral irritability, locomotion, attention, the response to stress, sleep and the startle response.
Activity of the LC mirrors the degree of arousal (i.e., sleep, calm-alert, alarm-vigilant, fear and terror) related to stress or distress in the environment (internal and external). Fear increases LC and VTN activity, increasing the release of norepinephrine in all of the LC and VTN terminal fields throughout the brain. The LC tunes out non-critical information and mediates hypervigilance. This nucleus orchestrates the complex interactive process that includes activation of autonomic nervous system tone, the immune system, the hypothalamic-pituitary-adrenal (HPA) axis with resulting release in adrenocorticotropin and cortisol. The sympathetic nervous system activation can be regulated by the LC, resulting in changes in heart rate, blood pressure, respiratory rate, glucose mobilization and muscle tone. All of these actions prepare the body for defense - to fight with or run away from the potential threat.
Hippocampus: Another key system linked with the RAS and playing a central role in the fear response is the hippocampus, located at the interface between the cortex and the lower diencephalic areas. It plays a major role in memory and learning. In addition it plays a key role in various activities of the autonomic nervous and neuroendocrine systems. Stress hormones and stress-related neurotransmitter systems (i.e., those from the locus coeruleus and other key brainstem nuclei) have the hippocampus as a target. In animal models, various hormones (e.g., cortisol) appear to alter hippocampus synapse formation and dendritic structure, thereby causing actual changes in gross structure and hippocampal volume as defined using various brain imaging techniques (see McEwen, 1999 for review). Repeated stress appears to inhibit the development of neurons in the dentate gyrus (part of the hippocampus) and atrophy of dendrites in the CA3 region of the hippocampus (Sapolsky & Plotsky, 1990; Sapolsky et al., 1990). These neurobiological changes are likely related to some of the observed functional problems with memory and learning that accompany stress-related neuropsychiatric syndromes, including post-traumatic stress disorder (PTSD: see Perry & Azad, 1999).
Amygdala and Emotional Memory: In the recent past, the amygdala has emerged as the key brain region in the processing, interpreting and integration of emotional functioning (Davis, 1992b). In the same fashion that the LC plays the central role in orchestrating arousal, the amygdala plays the central role in the CNS in processing afferent and efferent connections related to emotional functioning (Sapolsky, et al., 1984; Phillips & LeDoux, 1992). The amygdala receives input directly from sensory thalamus, hippocampus (via multiple projections), entorhinal cortex, sensory association areas of cortex, polymodal sensory association areas of cortex, and from various midbrain and brainstem arousal systems via the RAS (Selden et al., 1991). The amygdala processes and determines the emotional value of simple sensory input, complex multisensory perceptions and complex cognitive abstractions, even responding specifically to complex, socially relevant stimuli. In turn, the amygdala orchestrates the response to this emotional information by sending projections to brain areas involved in motor (behavioral), autonomic nervous system and neuroendocrine area of the CNS (Davis. 1992a; Davis. 1992a; LeDoux et al, 1988a). In a series of landmark studies, LeDoux and colleagues have demonstrated the key role of amygdala in emotional memory (LeDoux et al., 1990; LeDoux et al., 1989). In the response to threat, therefore, the amygdala and its related neural systems will have alterations in activity relative to the non-threat state.
Hypothalamic-Pituitary-Adrenal Axis (HPA) : As with central neurobiological systems, stress, threat and fear influence HPA regulation. Abnormalities of the HPA axis have been noted in adults with PTSD (Murberg et al., 1990). Chronic activation of the HPA system in response to stress has negative consequences. The homeostatic state associated with chronic HPA activation wears the body out (Sapolsky & Plotsky, 1990; Sapolsky et al., 1990). Hippocampal damage, impaired glucose utilization and vulnerability to metabolic insults may all result from chronic stress (see McEwen, 1999 for review).
The Dissociative Continuum
Infants and young children are not capable of effectively fighting or fleeing. In the initial stages of distress an infant will manifest a precursor form of a hyperarousal response. In these early alarm stages, the infant will use his limited behavioral repertoire to attract the attention of a caregiver. These behaviors include changes in facial expression, body movements and, most important, vocalization, i.e., crying. This is a successful adaptive strategy if the caretaker comes to feed, warm, and sooth, fight for, or flee with, the infant.
Unfortunately, for many infants and children these strategies are not effective. In the absence of an appropriate caregiver reaction to the initial alarm outcry, the child will abandon the early alarm response. The converse of use-dependent development occurs disuse related extinction of a behavior. This defeat response is well characterized in animal models of stress reactivity and learned helplessness (Miczek et al., 1990). This defeat reaction is a common element of the presenting emotional and behavioral phenomenology of many neglected and abused children (Spitz, 1945; George & Main, 1979; Carlson et al., 1994; Chisholm et al., 1995). Indeed, adults, professional or not, often puzzle over the emotional non-reactivity, passivity, compliance and hypalgesia of many abused children.
In the face of persisting threat, the infant or young child will activate other neurophysiological and functional responses. This involves activation of dissociative adaptations. Dissociation is a broad descriptive term that includes a variety of mental mechanism involved in disengaging from the external world and attending to stimuli in the internal world. This can involve distraction, avoidance, numbing, daydreaming, fugue, fantasy, derealization, depersonalization and, in the extreme, fainting or catatonia. In our experiences with young children and infants, the predominant adaptive responses during the trauma are dissociative.
Children exposed to chronic violence may report a variety of dissociative experiences. Children describe going to a 'different place', assuming the persona of superheroes or animals, a sense of watching a movie that I was in or just floating - classic depersonalization and derealization responses. Observers will report these children as numb, robotic, non-reactive, "day dreaming", "acting like he was not there", staring off in a glazed look. Younger children are more likely to use dissociative adaptations. Immobilization, inescapability or pain will increase the dissociative components of the stress response patterns at any age.
Neurobiology of dissociation
In animals, the defeat response is mediated by different neurobiological mechanism than the fight or flight response. What little is known about the neurobiology and phenomenology of dissociative-like conditions appears to most approximate the defeat reaction described in animals (Blanchard et al, 1993; Henry et al., 1986; Miczek et al., 1990). As with the hyperarousal response, there is brainstem mediated CNS activation that results in increases in circulating epinephrine and associated stress steroids. A major difference in the CNS, however, is that vagal tone increases dramatically, decreasing blood pressure and heart rate (occasionally resulting in fainting) despite increases in circulating epinephrine.
Dopaminergic systems, primarily mesolimbic and mesocortical, play an important role in defeat reaction models in animals. These dopaminergic systems are intimately involved in the reward systems, affect modulation (e.g., cocaine-induced euphoria) and, in some cases, are co-localized with endogenous opioids mediating pain or other sensory processing. The opioid systems are clearly involved in altering perception of painful stimuli, sense of time, place and reality . Opioids appear to be major mediators of the defeat reaction s dissociative behaviors (e.g., Abercrombie & Jacobs, 1988). Indeed, most opiate agonists can induce dissociative responses in humans.
The capacity to dissociate in the midst of terror appears to be a differentially available adaptive response - some people dissociate early in the arousal continuum, some only in a state of complete terror (see Table 1). The determinants of individual differences in the specific stress response to threat have yet to be well characterized. In its most common form, however, the child and adult response to trauma is an admixture of these two primary adaptive patterns, arousal and dissociation.
States become Traits: The Clinical Presentation of Children Exposed to Violence
A current working hypothesis regarding the effects of traumatic events on the neurobiology of the developing child posits that the specific symptoms a child develops will be related to the intensity and duration of the adaptive style (or combination of adaptive responses) present during the threat. If the neurobiology of the specific response (hyperarousal or dissociation) is activated long enough, there will be molecular, structural and functional changes in those systems (Perry, 1994; Perry et al., 1995; Perry, 1997; Perry & Pollard, 1998). Any factors that prolong the original threat response will increase the likelihood of long-term symptoms, while any factors that decrease the threat response will decrease risk for long-term problems.
If a child dissociates in response to a severe trauma and stays in that dissociative state for a sufficient period of time, she will alter the homeostasis of the systems mediating the dissociative response (i.e., opioid, dopaminergic, HPA axis). A sensitized neurobiology of dissociation will result and she may develop prominent dissociative-related symptoms (e.g., withdrawal, somatic complaints, dissociation, anxiety, helplessness, dependence) and related disorders (e.g., dissociative disorders, somatoform disorder, anxiety disorders, major depression).
If the child exposed to violence uses a predominately hyperarousal response, the altered homeostasis will be in different neurochemical systems (i.e., adrenergic, noradrenergic, HPA axis). This child will be vulnerable to developing persisting hyperarousal related symptoms and related disorders (e.g., PTSD, ADHD, conduct disorder). These children are characterized by persisting physiological hyperarousal and hyperactivity (Perry, 1995a; Perry, et al., 1995). They are observed to have increased muscle tone, frequently a low grade increase in temperature, an increased startle response, profound sleep disturbances, affect regulation problems and generalized (or specific) anxiety (Kaufman, 1991; Ornitz et al., 1989; Perry, 1994a). In addition, our studies indicate that a significant portion of these children have abnormalities in cardiovascular regulation (Perry, 1994; Perry et al., 1995b; see Figure 2).
The specific symptoms a child develops following exposure to violence, then, can vary depending upon the nature, frequency, pattern and intensity of the violence, the adaptive style of the child and the presence of attenuating factors such as a stable, safe and supportive home. Within this heterogeneity, however, certain trends emerge. Observations from clinical work suggest that there are marked gender differences in the response to violence (Perry et al., 1995b; Perry et al., 1995). Females are more likely to dissociate and males more likely to display a classic "fight or flight" response. As a result, more males will develop the aggressive, impulsive, reactive and hyperactive symptom presentation (more externalizing), while females will be more anxious, dissociative and dysphoric (more internalizing).
Children raised with persisting violence are much more likely to be violent (e.g., Loeber et al., 1993; Lewis et al., 1989; Koop et al., 1992; Hickey, 1991; Halperin et al., 1995). This can be explained, in part, by the persistence of this "fight or flight" state -- and by the profound cognitive distortions that can accompany a persisting state of fear. A young man with these characteristics may misinterpret a behavior as threatening and will, being more reactive, respond in a more impulsive and violent fashion. Literally, using the original (childhood) adaptive "fight or flight" response in a new context but, now, later in life, in a maladaptive fashion.
Altered Neurobiology in Children Exposed to Violence
Few studies have examined the neurobiological impact of trauma and violence in children. Several studies have utilized brain-regulated peripheral measures including psychophysiology (e.g., startle, heart rate regulation) or peripheral measures related to catecholamine or neuroendocrine functioning. In all of these studies, the findings have suggested a dysregulated, sensitized stress-response neurobiology in children and adolescents following exposure to trauma or violence (for review see Perry & Pollard, 1998; Perry & Azad, 1999). These findings are consistent with the hypothesis that the original adaptive neurophysiological states associated with the response to threat become, over time, in a use-dependent fashion, traits (Perry et al., 1995).
In one of the first studies to examine brain-related physiological responses in traumatized children, Ornitz and Pynoos (1989) demonstrated increased startle response, a finding suggesting sensitized brainstem and midbrain catecholamines (Davis, 1992). Similar altered brainstem catecholamine and neuroendocrine functioning was suggested by a pilot study in sexual abused girls. Following abuse girls exhibited greater total catecholamine synthesis as measured by the sum of the urinary concentration of epinephrine, norepinephrine and dopamine when compared with matched controls (DeBellis et al., 1994a; 1994b). In our laboratory, altered platelet alpha-2 adrenergic receptor number and cardiovascular functioning was demonstrated in children exposed to traumatic violence, suggesting chronic and abnormal activation of the sympathetic nervous system (Perry, 1994; Perry et al., 1995b). In our clinic populations, evidence of brain-mediated alterations of cardiovascular functioning have been demonstrated in various ways (Figures 1 and 2). In both the acute and chronic post-traumatic period, resting heart rate is different from comparison populations. In other studies, clonidine, an alpha2 adrenergic receptor partial agonist has been demonstrated to be an effective pharmacotherapeutic agent (Perry, 1994), further suggesting altered LC functioning in children exposed to violence.
Little research on the neurobiology of dissociation in children exists. In our preliminary studies, traumatized children with dissociative symptoms demonstrated lower heart rates than comparison-traumatized children with hyperarousal symptoms. Using continuous heart rate monitoring during clinical interviews, male, pre-adolescent children exposed to violence exhibited a mild tachycardia during non-intrusive interview and a marked tachycardia during interviews about specific exposure to trauma (n = 83; resting heart rate = 104; interview heart rate = 122). In comparison, females exposed to traumatic events tended to have normal or mild tachycardia that, during interviews about the traumatic event decreased (n =24; resting heart rate = 98; interview heart rate = 82). This gender difference was associated by differences in emotional and behavioral symptoms, with males exhibiting more externalizing and females more internalizing symptoms (Perry, et al., 1995b; see Figure 3). In a recent case series with ten children suffering from severe dissociative symptoms (e.g., fainting, catatonia, bradycardia) naltrexone, an opioid antagonist, improved dissociative symptoms (Perry et al., in preparation). The hypothesized therapeutic site of action is the opioid receptors regulating LC activity (Abercrombie and Jacobs, 1998).
These indirect studies all support the hypotheses of use-dependent alterations in the key neural systems of the brain related to the stress-response following exposure to violence in childhood. More recently, using newer methods allowing more direct examination of the brain supports the notion that prolonged threat alters the developing brain. Preliminary studies by Teicher and colleagues have demonstrated altered EEG findings in a sample of abused children suggest hippocampal/limbic and cortical abnormalities (Ito et al., 1993; Teicher et al., 1997). DeBellis in a series of landmark studies (1999a; 1999b) demonstrated altered cortical development in children with PTSD. In 44 PTSD subjects (1999a), the intracranial and cerebral volumes were smaller than matched controls. These differences were related to the severity and onset of symptoms.
Clearly more research is indicated, however, all studies to date suggest that exposure to violence in childhood alters brain development and that the abnormalities are more prominent if the traumatic exposure is early in life, severe and chronic.
Clinical Implications
There are profound clinical implications of the persisting fear states in children. These children will have impaired capacities to benefit from social, emotional and cognitive experiences. This is explained by three key principles of brain functioning: 1) the brain changes in response to experience in a use-dependent fashion; 2) the brain internalizes and stores information from any experience in a state-dependent fashion and 3) the brain retrieves stored information in a state-dependent fashion.
Use-dependent Learning: State Dependent Storage and Recall
As described above, the brain changes in a use-dependent fashion. All parts of the brain can modify their functioning in response to specific patterns of activation. These use-dependent changes in the brain result in changes in cognition (this, of course, is the basis for cognitive learning), emotional functioning (social learning), motor-vestibular functioning (e.g., the ability to write, type, ride a bike) and state-regulation capacity (e.g., resting heart rate). No part of the brain can change without being activated -- you can t teach someone French while they are asleep or teach a child to ride a bike by talking with him.
One of the most important elements of understanding children exposed to violence is that all humans process, store, retrieve and respond to the world in a state-dependent fashion (see Table 1). When a child is in a persisting state of low-level fear that results from exposure to violence, the primary areas of the brain that are processing information are different from those in a child from a safe environment. The calm child may sit in the same classroom next to the child in an alarm state, both hearing the same lecture by the teacher. Even if they have identical IQs, the child that is calm can focus on the words of the teacher and, using neocortex, engage in abstract cognition. The child in an alarm state will be less efficient at processing and storing the verbal information the teacher is providing. This child s cognition will be dominated by sub-cortical and limbic areas, focusing on non-verbal information - the teacher s facial expressions, hand gestures, when she seems distracted. And, because the brain internalizes (i.e., learns) in a use-dependent fashion, this child will have more selective development of non-verbal cognitive capacities. The children raised in the vortex of violence have learned that non-verbal information is more important than verbal.
This means that hypervigilant children from chronic violence settings frequently develop remarkable non-verbal skills in proportion to their verbal skills (street smarts). Indeed, often they over-read (misinterpret) non-verbal cues; eye contact means threat, a friendly touch is interpreted as an antecedent to seduction and rape, accurate in the world they came from but now, hopefully, out of context. During development, these children spent so much time in a low-level state of fear (mediated by brainstem and midbrain areas) that they were focusing consistently on non-verbal cues. In our clinic population, children raised in chronically traumatic environments demonstrate a prominent V-P split on IQ testing (n = 108; WISC Verbal = 8.2; WISC Performance = 10.4, Perry, in preparation). In a separate study of 400 children removed from their parents by child protective services, IQ testing demonstrated that only 2 % of the children had a significant Verbal>Performance split (V score 12 points greater than P score) while 39 % demonstrated a significant P>V split (P score 12 points greater than V score) (Perry et al. in preparation).
This is consistent with the observations of teachers that many of the maltreated or traumatized children they work with are often judged to be bright but can t learn easily. Often these children are labeled as learning disabled. These difficulties with cognitive organization contribute to a more primitive, less mature style of problem solving - with aggression often being employed as a "tool".
This principle is critically important in understanding why a traumatized child - in a persisting state of arousal - can sit in a classroom and not learn. The brain of this child has different parts of the brain controlling his functioning than a child that is calm. The capacity to internalize new verbal cognitive information depends upon having portions of the frontal and related cortical areas being activated. This, in turn, requires a state of attentive calm. A state the traumatized child rarely achieves.
Children in a state of fear retrieve information from the world differently than children that feel calm (see Table 1). As a child moves along the continuum of arousal (see Table 1), the part of the brain that is orchestrating functioning shifts. An important reflection of this is how the sense of time is altered in alarm states. Sense of future is foreshortened; the critical time period for the individual shrinks. The threatened child is not thinking (nor should she think) about months from now. This has profound implications for understanding the cognition of the traumatized child. Immediate reward is most reinforcing. Delayed gratification is impossible. Consequences of behavior become almost inconceivable to the threatened child. Reflection on behavior -including violent behavior - is impossible for the child in an alarm state. Cut adrift from internal regulating capabilities of the cortex, the brainstem acts reflexively, impulsively, and aggressively to any perceived threat. Eye contact for too long becomes a life-threatening signal. Wearing the wrong colors - a hand gesture - cues that to the calm adult reading about another senseless murder in the paper are insignificant but to the hypervigilant, armed adolescent born and raised in the vortex of violence, enough to trigger a kill or be killed response.
The Future: Impediments to Problem-Solving and Prevention
There are many important and effective treatment approaches to the child traumatized by violence. Yet even with optimal clinical techniques , treatment of maltreated children would overwhelm the entire mental health and child welfare community in this country. Today the number of children that would benefit from intervention far outstrips the meager resources our society has dedicated to children exposed to violence. Even as we develop more effective and accessible intervention models, we must focus on prevention.
A society functions as a reflection of its childrearing practices. If children are ignored, poorly educated and not protected from violence they will grow into adults that create a reactive, non-creative and violent society. In a brilliant analysis of this very process, Hellie (1996) describes a dark age in Russia (1600 to 1700) characterized by excessive brutality, violence and pervasive fear that for generations inhibited creativity, abstraction, literacy and the other elements of humanity. All societies reap what they have sown.
Today, in the United States, despite the well-documented adverse effects of domestic, community, school and media violence, we continue to seek short-term and simplistic answers. In order to minimize the many destructive pathways that come from violence in childhood, we need to dedicate resources of time, energy and money to these complex problems. And we need to help provide the resource-predictable, safe and resource rich environments our problem-solvers require. Too often the academic, public and non-for-profit systems asked to address these problems are resource-depleted yet have a mandate to "do something." Unfortunately, the solutions that arise from this reactive approach to complex problems are very limited and, typically, short-sighted (see Table 2).
Our problem-solvers must understand the indelible relationship between early life experiences and cognitive, social, emotional, and physical health. Providing enriching cognitive, emotional, social and physical experiences in childhood could transform our culture. But before our society can choose to provide these experiences, it must be educated about what we now know about child development. Education of the public must be coupled with the continuing research into the impact of positive and negative experiences on the development of children. All of this must be paired with the implementation and testing of programs that can enrich the lives of children and families and programs to provide early identification of, and proactive intervention for, at-risk children and families.
The problems related to violence are complex and they have complex impact on our society. Yet there are solutions to these problems. The choice to find solutions is up to us. If we choose, we have some control of our future. If we, as a society, continue to ignore the laws of biology, and the inevitable neurodevelopmental consequences of chronic exposure to violence in childhood, our potential as a humane society will remain unrealized. The future will hold sociocultural devolution - the inevitable consequence of the competition for limited resources and the implementation of reactive, one-dimensional and short-term solutions. This need not be. Parents, caregivers, professionals, public officials and policy makers do have the capacity to make decisions that will increase or decrease violence in our children s lives. Hopefully, an appreciation of the devastating impact of violence on the developing child will help all of us make the good decisions and difficult choices that will create a safer, more predictable and enriching world for children.
References
Abercrombie ED, Jacobs BL: Single-unit response of noradrenergic neurons in the locus coeruleus of freely moving cats . I. Acutely presented stressful and non-stressful stimuli. J Neurosci 7:2837-2843, 1987a
Abercrombie ED, Jacobs BL: Single-unit response of noradrenergic neurons in the locus coeruleus of freely moving cats. II. Adaptation to chronically presented stressful stimuli. J Neurosci 7:2844-2848, 1987b
Abercrombie ED, Jacobs BL: Systemic naloxone administration potentiates locus coeruleus noradrenergic neuronal activity under stressful but not non-stressful conditions. Brain Research 441:362-366, 1988
Aston-Jones G, Valentino RJ, Van Bockstaele EJ, et al: Locus coeruleus, stress and post traumatic stress disorder: neurobiological and clinical parallels. In M. Murberg (Ed.), Catecholamine function in post traumatic stress disorder: emerging concepts.Washington D.C. American Psychiatric Press, 1996
Belmore MF, Quinsey VL: Correlates of psychopathy in a noninstitutional sample. Journal of Interpersonal Violence 9(3):339-349, 1994
Blanchard DC, Sakai RR, McEwen B, et al: Subordination stress: behavioral, brain and neuroendocrine correlates. Behavioral Brain Research 58:113-121, 1993
Brown JW: Morphogenesis and mental process. Development and Psychopathology 6:551-563, 1994
Burton J, Foy D, Bwanausi C, et al: The relationship between the traumatic exposure, family dysfunction and post-traumatic stress symptoms in male juvenile offenders. Journal of Traumatic Stress 7:83-93, 1994
Carlson V, Cicchetti D, Barnett D, et al: Disorganized/disoriented attachment relationships in maltreated infants. Developmental Psychology 25:525-531, 1989
Carnegie Council on Adolescent Development. Great Transitions: Preparing Adolescents for a New Century. New York: Carnegie Corporation of New York, 1995
Chisholm K, Carter MC, Ames EW, et al: Attachment security and indiscriminately friendly behavior in children adopted from Romanian orphanages. Development and Psychopathology 7:283-294, 1995
Comstock GA, Paik HJ: Television and the American Child. Academic Press San Diego, CA, 1991
Courchesne E, Chisum H, Townsend J: Neural activity-dependent brain changes in development: implications for psychopathology. Development and Psychopathology 6(4):697-722, 1994
Davis M: The role of the amygdala is fear-potentiated startle: implications for animal models of anxiety. Trends Pharmacol Sci 13:35-41, 1992a
Davis M: The role of the amygdala in conditioned fear. In J.P. Aggleton (Ed.), The amygdala: neurobiological aspects of emotion, memory, and mental dysfunction.New York: Wiley-Liss, 1992b, pp 255-306
De Bellis MD, Chrousos GP, Dorn LD, et al: Hypothalamic-pituitary-adrenal axis dysregulation in sexually abused girls. Journal of Clinical Endocrinology and Metabolism 78:249-255, 1994
De Bellis MD, Lefter L, Trickett PK, et al: Urinary catecholamine excretion in sexually abused girls. Journal of the American Academy of Child and Adolescent Psychiatry 33:320-327, 1994
DeBellis MD, Baum AS, Birmaher B, et al: Developmental traumatology part I: biological stress symptoms. Biological Psychiatry 45:1259-1270, 1999
DeBellis MD, Keshavan MS, Clark DB, et al: Developmental traumatology part II: brain development. Biological Psychiatry 45:1271-1284, 1999
Dodge KA, Bates JE, Pettit GS: Mechanisms in the cycle of violence. Science 250:1678-1683, 1991
Donnerstein E, Slaby R, Eron L: The mass media and youth aggression. In L. Eron, J. Gentry, & P. Schlegel (Eds.), Reason to Hope: a psychosocial perspective on violence and youth. Washington, D.C. American Psychological Association, 1995
Garbarino J: Children's response to community violence: what do we know? Infant Mental Health Journal 14:103-115, 1993
Garrity C, et al: Bully-proofing Your School: A Comprehensive Approach for Elementary School. Sopris West Press, Longmont, CO, 1995
George C, Main M: Social interactions of young abused children: approach, avoidance and aggression. Child Development 50:306-318, 1979
Gerbner G: Society s storyteller: How television creates the myths by which we live. Media & Values 59:8-9, 1992
Green AH, Voeller K, Gaines RW, et.al: Neurological impairment in maltreated children. Child Abuse and Neglect 5:129-134, 1981
Greenberg MT, Speltz ML, DeKlyen M: The role of attachment in the early development of disruptive behavior problems. Development and Psychopathology 5:191-213, 1993
Halperin JM, Newcorn JH, Matier K: Impulsivity and the initiation of fights in children with disruptive behavioral disorders. J Child Psychol Psychiat 36(7):1199-1211, 1995
Hellie R: Interpreting violence in late Muscovy from the perspective of modern neuroscience. Presented paper, 28th National Covention of the AAAS, Boston, 1996
Henry JP, Liu YY, Nadra WE, et al: Psychosocial stress can induce chronic hypertension in normotensive strains of rats. Hypertension 21:714-723, 1993
Hickey E: Serial Murderers and Their Victims. Belmont, CA: Wadsworth Publishing, 1991
Horowitz K, Weine S, Jekel J: PTSD symptoms in urban adolescent girls: compounded community trauma. Journal of the American Academy of Child and Adolescent Psychiatry 34(10):1353-1361, 1995
Huston AC, Donnerstein E, Fairchild H, et al: Big World, Small Screen: The Role of Television in American Society. Lincoln, NE: University of Nebraska Press, 1992.
Ito Y, Teicher MH, Glod CA, et.al: Increased prevalence of electrophysiological abnormalities in children with psychological, physical, and sexual abuse. Journal of Neuropsychiatry 5:401-408, 1993
Kaufman J: Depressive disorders in maltreated children. Journal of the American Academy of Child and Adolescent Psychiatry 30(2):257-265, 1991
Koop CE, Lundberg G: Violence in America: a public health emergency. Journal of the American Medical Association 22:3075-3076, 1992
Lauder JM: Neurotransmitters as morphogens. Progress in Brain Research 73:365-388, 1988
LeDoux JE, Cicchetti P, Xagoraris A, et al: The lateral amygdaloid nucleus: sensory interface of the amygdala in fear conditioning. J Neurosci 10:1062-1069, 1990
LeDoux JE, Romanski L, Xagoraris A: Indelibility of subcortical emotional memories. Journal of Cognitive Neuroscience 1:238-243, 1989
Lewis DO, Mallouh C, Webb V: Child abuse, delinquency, and violent criminality. In D. Chiccetti & V. Carlson (Eds.), Child Maltreatment: theory and research on the causes and consequences of child abuse and neglect. Cambridge: Cambridge University Press, 1989
Loeber R, Wung P, Keenan K, et al: Developmental pathways in disruptive child behavior. Development and Psychopathology 5:103-133, 1993
McAllister AK, Katz LC, Lo DC: Neurotrophins and synaptic plasticity. Annu Rev Neurosci 22:295-318, 1999
McEwen BS: Stress and hippocampal plasticity. Annu Rev Neurosci 22:105-122, 1999
Miczek KA, Thompson ML, Tornatzky W: Subordinate animals: Behavioral and physiological adaptations and opioid tolerance. In Stress: Neurobiology and Neuroendocrinology. Edited by MR Brown, GF Koob, C Rivier, New York, Marcel Dekker, 1990, pp 323-357
Mones P: When a Child Kills: abused children who kill their parents. New York: Pocket Books, 1991
Moore RY, Bloom FE: Central catecholamine neuron systems: anatomy and physiology of the norepinephrine and epinephrine systems. Annual Reviews of Neuroscience 2:113-153, 1979
Munk MHJ, Roelfsema PR, Konig P, et al: Role of Reticular Activation in the Modulation of Intracortical Synchronization. Science 272:271-273, 1996
Murberg MM, McFall ME, Veith RC: Catecholamines, stress and post-traumatic stress disorder. In E.L. Giller (Ed.), Biological Assessment and Treatment of Post-traumatic Stress Disorder.Washington, D.C. American Psychiatric Press, Inc., 1990, pp 27-65
Myers WC, Scott K, Burgess AW, et al: Psychopathology, biopsychosocial factors, crime characteristics and classification of 25 homicidal youths. Journal of the American Academy of Child and Adolescent Psychiatry 34(11):1483-1489, 1995
O'Keefe M: Predictors of child abuse in maritally violent families. Journal of Interpersonal Violence 10(1):3-25, 1995
Ornitz EM, Pynoos RS: Startle modulation in children with post-traumatic stress disorder. American Journal of Psychiatry 147:866-870, 1989
Perry BD: Neurobiological sequelae of childhood trauma: post-traumatic stress disorders in children. In M. Murberg (Ed.), Catecholamines in Post-traumatic Stress Disorder: Emerging Concepts. Washington, D.C. American Psychiatric Press, 1994a, pp 253-276
Perry BD, Pollard RA, Baker WL, et al: Continuous heartrate monitoring in maltreated children [Abstract]. Annual Meeting of the American Academy of Child and Adolescent Psychiatry, New Research, 1995
Perry BD, Pollard R, Blakely T, et al: Childhood trauma, the neurobiology of adaptation and 'use-dependent' development of the brain: how "states" become "traits'". Infant Mental Health Journal 16(4):271-291, 1995 (eine Übersetzung befindet sich auf der Webseite von Dr. Karl C. Mayer unter www.neuro24.de/ptbs2.htm)
Perry BD: Incubated in terror: neurodevelopmental factors in the cycle of violence In: Children, Youth and Violence: The Search for Solutions (J Osofsky, Ed.). Guilford Press, New York, 1997, pp 124-148
Perry BD: Memories of fear: how the brain stores and retrieves physiologic states, feelings, behaviors and thoughts from traumatic events:In: Splintered Reflections: Images of the Body in Trauma (JM Goodwin and R. Attias, Ed.). Basic Books, 1999, pp 26-47
Perry BD, Pollard R: Homeostasis, stress, trauma, and adaptation: a neurodevelopmental view of childhood trauma. Child and Adolescent Psychiatric Clinics of North America 7(1):33-51, 1998
Perry BD, Azad I: Post-traumatic stress disorders in children and adolescents. Current Opinion in Pediatrics 11:121-132, 1999
Phillips RG, LeDoux JE: Differential contribution of amygdala and hippocampus to cued and contextual fear conditioning. Behav Neurosci 106:274-285, 1992
Richters J, Martinez P: Violent communities, family choices and children s chances: an algorithm for improving the odds. Development and Psychopathology 5:609-627, 1993
Sapolsky RM, Plotsky PM: Hypercortisolism and its possible neural bases. Biological Psychiatry 27:937-952, 1990
Sapolsky RM, Uno H, Rebert CS, et.al.: Hippocampal damage associated with prolonged glucocorticoid exposure in primates. Journal of Neuroscience 10:2897-2902, 1990
Selden NRW, Everitt BJ, Jarrard LE: Complementary roles for the amygdala and hippocampus in aversive conditioning to explicit and contextual cues. Neuroscience 42:335-350, 1991
Spitz R: Hospitalism: an inquiry into the genesis of psychiatric conditions in early childhood. Psychoanalytic Study of the Child I:53-74, 1945
Straus M: Cultural and organizational influences on violence between family members. In R. Prince & D. Barried (Eds.), Configurations: Biological and Cultural Factors in Sexuality and Family Life. Washington,D.C. Health, 1974
Straus M, Gelles R: How violent are American families: estimates from the national family violence survey and other studies. In: Family Abuse and Its Consequences: New Directions in Research (G. Hotaling et al., Eds) Sage Press, Newbury Park, CA, 1998
Synder H: Juvenile arrests 1996: Juvenile Justice Bulletin Office of Juvenile Justice and Delinquency Prevention, US Department of Justice, Washington DC, 1997, pp 1-12
Taylor L, Zuckerman B, Harik V, et al: Exposure to violence among inner city parents and young children. American Journal of Diseases of Children, 146:487, 1992
Teicher M, Ito Y, Glod CA, et al: Preliminary evidence for abnormal cortical development in physically and sexually abused children using EEG coherence and MRI. In: Yehuda, R.M. (Ed.) Psychobiology of Post-traumatic Stress Disorder. Vol 821; Annals of the New York Academy of Science, New York, NY, 1997, pp 160-175
Vaid RR, Yee BK, Shalev U, et al: Neonatal nonhandling and In Utero prenatal stress reduce the density of NADPH-diaphorase-reactive neurons in the fascia denata and Ammon's Horn of rats. The Journal of Neuroscience 17:5599-5609, 1997
Vallee M, Mayo W, Dellu F, et al: Prenatal stress induces high anxiety and postnatal handling induces low anxiety in adult offspring: correlation with stress-inducing corticosterone secretion. The Journal of Neuroscience 17:2626-2636, 1997
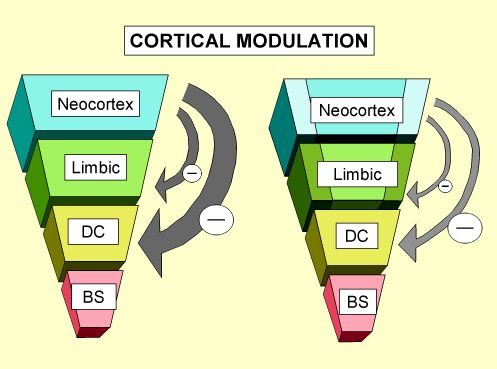 |
Figure 1. Cortical Modulation: As the brain develops in this sequential and hierarchical fashion, and the more complex limbic, sub-cortical and cortical areas organize, they begin to modulate, moderate and control the more primitive and reactive lower portions of the brain( DC: diencephalon; BS: brainstem). These various brain areas develop, organize and become fully functional at different times during childhood. At birth, for example, the brainstem areas responsible for regulating cardiovascular and respiratory function must be intact while the cortical areas responsible for abstract cognition have years before they will be fully functional.
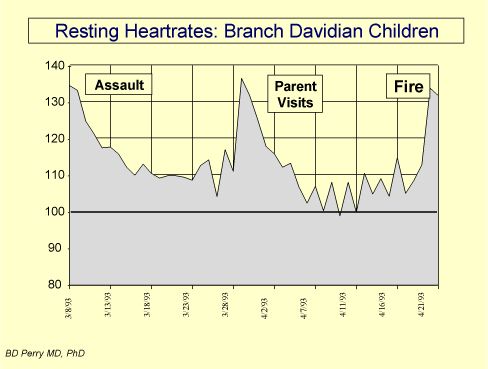 |
Figure 2. Hyperarousal symptoms following a life-threatening event: In the three days following the ATF assault on the Branch Davidian s Ranch Apocalypse compound, twenty one children were released. Each of these children were in harm s way during the assault. Following release, a clinical team led by ChildTrauma Program personnel lived and worked with these children for the next six weeks. These children had various PTSD-related symptoms. Re-enactment behaviors and cue-specific increases in anxiety were observed in the presence of cues associated with the assault, including white vans and a helicopter. The physiological hyperarousal was illustrated by the profound increases in resting heart rate observed in all of the children throughout the six weeks of the standoff. Five days after the original raid, the group average resting heart rate was 134 (the group average should have been approximately 80). In the middle of the stand off, many of these children visited with a parent released from the compound. These visits resulted in dramatic changes in the children s behavior (e.g., return of bed-wetting, hiding under beds, aggressive behavior) and in their resting heart rates, indicating that these visits were, in some regard, distressing to the children. During these visits, the children were reminded by their parent that they were in the hands of the Babylonians , inducing fear and confusion. When these visits stopped, the children improved. When the chidlren were told about the fire, as one would expect, their distress increased dramatically. It should be noted that the normal resting heart rate for a group of comparison children is approximately 90 beats per minute -- the Davidian children for the entire period of the stand off and beyond never had resting heart rates below 100.
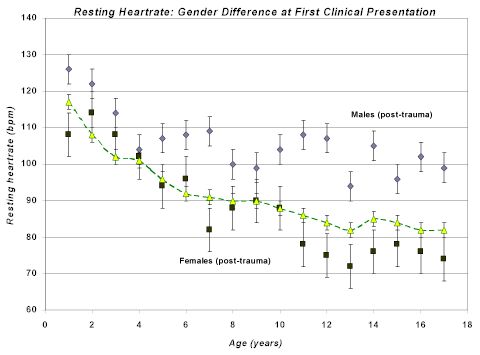 |
Figure 3. Resting heart rate in traumatized children. Over a five year period, each child referred to the ChildTrauma Clinic specializing in working with traumatized or maltreated children had a resting heart rate taken at first presentation. These resting rates were plotted by age and gender (total n=526; traumatized males are the grey diamonds +/- SEM, n=320; traumatized females are the black squares +/- SEM, n=206). The yellow diamonds are values from normal pediatric population norms in which there are no observed gender differences. In young children, there do not appear to be any gender differences; by age five, however, gender differences emerge with males having higher resting heart rate (consistent with persisting hyperarousal) and females having somewhat lower resting heart rate (consistent with persisting dissociative adaptations). These resting rates are pre-treatment.
|
Sense of Time
|
Extended Future
|
Days
Hours
|
Hours
Minutes
|
Minutes
Seconds
|
No Sense
Of Time
|
|
Arousal
Continuum
|
REST
|
VIGILANCE
|
RESISTANCE
Crying
|
DEFIANCE
Tantrums
|
AGGRESSION
|
|
Dissociative
Continuum
|
REST
|
AVOIDANCE
|
COMPLIANCE
Robotic
|
DISSOCIATION
Fetal Rocking
|
FAINTING
|
|
Regulating Brain Region
|
NEOCORTEX
Cortex
|
CORTEX
Limbic
|
LIMBIC
Midbrain
|
MIDBRAIN
Brainstem
|
BRAINSTEM
Autonomic
|
|
Cognitive Style
|
ABSTRACT
|
CONCRETE
|
EMOTIONAL
|
REACTIVE
|
REFLEXIVE
|
|
Internal State
|
CALM
|
AROUSAL
|
ALARM
|
FEAR
|
TERROR
|
|
Table 1. The continuum of adaptive responses to threat. Different children have different styles of adaptation to threat. Some children use a primary hyperarousal response, others a primary dissociative response. Most use some combination of these two adaptive styles. In the fearful child, a defiant stance is often seen. This is typically interpreted as a willful and controlling child. Rather than understanding the behavior as related to fear, adults often respond to the oppositional behavior by becoming angry and more demanding. The child, over-reading the non-verbal cues of the frustrated and angry adult, feels more threatened and moves from alarm to fear to terror. These children may end up in a primitive "mini-psychotic" regression or in a very combative state. The behavior of the child reflects their attempts to adapt and respond to a perceived (or misperceived) threat.
When threatened a child is likely to act in an immature fashion. Regression, a retreat to a less mature style of functioning and behavior, is commonly observed in all of us when we are physically ill, sleep-deprived, hungry, fatigued or threatened. During the regressive response to the real or perceived threat, less-complex brain areas mediate our behaviors. If a child has been raised in an environment of persisting threat, the child will have an altered baseline such that the internal state of calm is rarely obtained (or only artificially obtained via alcohol or drug use). In addition, the traumatized child will have a sensitized alarm response, over-reading verbal and non-verbal cues as threatening. This increased reactivity will result in dramatic changes in behavior in the face of seemingly minor provocative cues. All too often, this over-reading of threat will lead to a fight or flight reaction - and increase the probability of impulsive aggression. This hyper-reactivity to threat can, as the child becomes older, contribute to the transgenerational cycle of violence.
|
Social-
Environmental Pressures
|
Resource-surplus
Predictable
Stable/Safe
|
Resource-limited Unpredictable
Novel
|
Resource-poor Inconsistent
Threatening
|
|
Prevailing
Cognitive Style
|
Abstract
Creative
|
Concrete
Superstitious
|
Reactive
Regressive
|
|
Prevailing
Affective ‘Tone’
|
CALM
|
ANXIETY
|
TERROR
|
|
Systemic Solutions
|
INNOVATIVE
|
SIMPLISTIC
|
REACTIONARY
|
|
Focus of Solution
|
FUTURE
|
Immediate FUTURE
|
PRESENT
|
|
Rules, Regulations and Laws
|
Abstract
Conceptual
|
Superstitious
Intrusive
|
Restrictive
Punitive
|
|
Childrearing
Practices
|
Nurturing
Flexible
Enriching
|
Ambivalent
Obsessive
Controlling
|
Apathetic
Oppressive
Harsh
|
|
Table 2. The continuum of adaptive responses to threat in a living group (family, organization, community or society). In the same fashion as an individual, living groups experience threat and challenges to their survival. Similar to an individual, the cognition of a group moves down a gradient under threat. When there are no external threats and resources are plentiful and predictable (Column 1), the group has the luxury of thinking in abstract ways to solve any of its current problems (e.g., Bell Labs from 1940 to 1965). The focus of the solution can be the future and the least powerful members of the living group (e.g., children and women) can be treated with the most flexible, nurturing and enriching approaches. When resources become limited and there are economic, environmental or social threats (Column 2), the group, organization or society become less capable of complex, abstract problems solving. The solutions tend to reflect the immediate future (e.g., the next funding cycle, the next election cycle) and all aspects of functioning in the group become more regressed. The least powerful are ignored or controlled to minimize any excessive drain on the most powerful. In a group, organization or society under direct threat (Column 3), the focus of all problem solving becomes the moment. The solutions tend to be reactive and regressive. The least powerful are ignored and, if they get in the way, they are harshly dealt with. The more out of control the external situation is, the more controlling, reactive and oppressive the internally focused actions of this group will become. In each of these situations, the prevailing childrearing styles will create children that will reinforce that group or society s structure: in a safe and abstract-thinking group the children will be more likely to receive and benefit from enrichment and education, thereby optimizing their potential for creativity, abstraction and productivity. In contrast, children raised in groups or societies under threat will be more likely to be raised with harsh or distant caregiving. The result will be impulsive, concrete and reactive adults, perfectly positioned to fit in and contribute to a reactive, oppressive and aggressive group or society.
In: childtrauma.org (s. Kontaktadressen)
inzwischen als Beitrag in: Perry, B.D. (2001b). The neurodevelopmental impact of violence in childhood. In Schetky D. & Benedek, E. (Eds.) Textbook of child and adolescent forensic psychiatry. Washington, D.C.: American Psychiatric Press, Inc. (221-238)
weitere Beiträge zum Thema Traumaforschung
|